Congenital birth defects may be treated by using amniotic fluid, the protective liquid surrounding an unborn baby often discarded as waste during caesarean deliveries . Over the past decade, studies have shown that this fluid is a valuable source for deriving stem cells and is an emerging possibility for Cell Therapy or Regenerative Medicine. Amniotic fluid is also a source for Mesenchymal Stem Cells (MSCs), also called a multipotent tissue or adult stem cell which can produce more than one type of specialized cell, useful in treating different diseases such as cardiovascular disease, diabetes, arthritis, and neurodegenerative disorders.
According to the research by Shuan M. Kunisaki, Amniotic Fluid Stem Cells for the Treatment of Surgical Disorders in the Fetus and Neonate, amniotic fluid-derived stem cells have been the source of inspiration for experimental approach which aims at improving treatment for birth defects in a newborn. Popularity of the study of these fetus cells, also called amniocytes, for prenatally diagnosed diseases were based on several studies that prove its unique properties and their beneficial effects tested on animals models.Some of the emerging Cell Therapy resulting from the study of amniotic fluid, includes spina bifida treatment and treatment for birth heart defects. Currently, amniotic fluid based-stem cell technologies has been used with the fabrication of tissue-engineered heart valves which could lead to treatment for birth heart defects.
There are several reasons why amniotic fluid-derived stem cells are becoming one of the breakthrough approaches
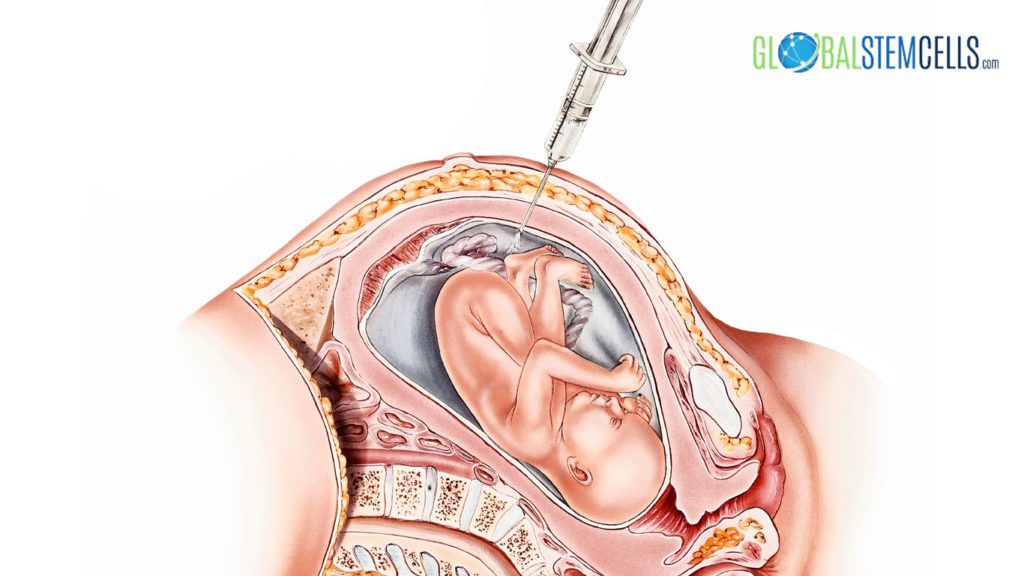
in treating children with congenital anomalies or birth defects. According to Kunisaki’s thesis, one of the reason is that there is no need to wait until delivery for cell harvesting since amniocytes are easily accessible through the process of amniotic fluid test. During this process, a small amount of amniotic fluid is removed from the sac surrounding the fetus for testing. Contrasted to the traditional method of harvesting stem cells prenatally from placenta, chorionic villi, cord blood, liver or skin, the amniotic fluid test procedure is less challenging and has smaller risks of abortion, infection, hemorrhage, and other morbidities.
Another fact amniotic fluid-derived stem cells is gaining more attention is that MSCs can be obtained from other tissues in the body, however, the difficulty in acquiring an abundant number of cells limits its use in Cell Therapy and tissue repair applications, according to Lund University. “Full term amniotic fluid, being an easily obtainable and abundant tissue source, may be the solution for MSC based cell therapy and regenerative medicine applications”, said Associate Professor Niels-Bjarne Woods, a corresponding author of a study done by scientists and clinicians at Lund University in Sweden. Amniotic fluid-derived stem cells comes from the fetus itself, therefore it can be used in Stem Cell Therapy without the concern for immunologic rejection of donor cells or the rejection by the recipient’s immune system. In fact, Kunisaki wrote that the safety with amniocentesis is causing a rise in commercial banking of amniotic fluid in developed countries.
H/T: Lund University



 English
English