
Unique Access provides access to an extensive treatment protocol for Muscular Dystrophy which uses higher quantities of stem cells, extensive rehabilitation, and many supportive therapies. This effective combination of the most advanced medical technologies, with systematic physical and occupational therapies, has helped previously treated patients achieve a broad range of important improvements.
Why Stem Cells Work for Muscular Dystrophy
Different types of stem cells, including bone marrow-derived stem cell, Umbilical Cord Mesenchymal Stem Cell (UCMSC), Umbilical Cord Blood Stem Cells, have been under research as novel options to treat Duchenne Muscular Dystrophy (DMD) both on disease models and clinical trials in recent years.
Mechanisms
Mesenchymal Stem Cells (MSC) can help relieve the disease symptoms through the following mechanisms, based on their uniquely different characteristics:
1. MSCs can lead to skeletal muscle regeneration. MSCs transplantation may ameliorate patient’s clinical symptoms through defective gene correction, and muscle cell regeneration
2. MSCs lead to an apparent therapeutic benefit in disease models through their immunomodulatory effect, which is another underlying mechanism of Mesenchymal Stem Cells (MSCs) for treating Muscular Dystrophy (MD).
3. MSCs possess myotube generation and dystrophin expression potentials leading to muscle strength increment in patients.
Possible Improvements
Most of the Muscular Dystrophy (MD) patients Unique Access has helped receive innovative treatments, using the combination of Umbilical Cord Mesenchymal Stem Cell (UCMSC), supportive therapies and rehabilitation, showed remarkable improvements. These improvements included increased muscle mass and strength, improved motor function, improved balance and coordination, decreased occurrence of respiratory infections and secondary complications and maintained clinical stabilisation, or some of the patients have even walked after the treatment. Moreover, the disease progression has reposted to be significantly slower after stem cell treatment.
Muscular Dystrophy (MD) patients treated with stem cells usually observe improvements in the following areas:
- Increased muscle mass and strength
- Motor function
- Balance and coordination
- Slowed down disease progression
Our Promise
We believe that there is always hope and that patients deserve access to effective and safe treatments. We are independent with an in-house medical department. We combine internationally accredited hospitals, next generation treatments, unique products and services that are integrative and effective to ensure best possible treatment results.
Why Stem Cells Treatment is Ideal for Muscular Dystrophy Patients
Unique Access has successfully helped patients to receive innovative treatments with various types of Muscular Dystrophies with a combination of Mesenchymal Stem Cells (MSC), immune boost, diet and extensive rehabilitation.
MSCs are well known for differentiating into multiple cell types including muscle fibres and relatively easier success to affected muscles (by intravenous and intramuscular transplantations) makes Muscular Dystrophy one of the best stem cell treatment candidates.
Stem Cells
In terms of stem cells we will make sure that the patient will receive the correct and necessary stem cell type, quality, quantity and viability. Our exclusive research partner guarantee a stem cell viability of 95%, many injections have a staggering viability of 98-99%.
Supportive Therapies & Remedies
- Hyperbaric Oxygen Chamber (HBOT)
- Hemo Oxygen Therapy (HOT)
- IV Vitamin Drips
- Immune-Boosting Supplements (e.g. GcMAF)
Partner Hospital
The treatment will take place in an internationally accredited tertiary care hospital and not in a hotel or clinic. This is important for the patient’s safety and care as the patient will have access to all specialized departments & specialist doctors which will further increase the treatments efficiency.
What is Muscular Dystrophy?
Muscular Dystrophy (MD) is a group of inherited progressive muscle disorders which are characterised by muscle weakness, wasting and degeneration. Some forms of Muscular Dystrophy (MD) can also affect other body organs such as heart, gastrointestinal tract and the brain. Muscular Dystrophy (MD) are caused by mutations in genes that code for specific muscle proteins.
Duchenne Muscular Dystrophy (DMD) is one of the most common and lethal Muscular Dystrophys which affects 1 in every 3,500 males and is caused by mutations in the dystrophin gene. Dystrophin is a critical component of the Dystrophin Glycoprotein Complex (DGC), which is involved in stabilising interactions between the sarcolemma, the cytoskeleton, and the extracellular matrix of skeletal and cardiac muscles. There are many other kinds of Muscular Dystrophies (MDs), including Becker’s Muscular Dystrophy, Limb Girdle Muscular Dystrophy and Facioscapulohumeral Muscular Dystrophy, etc.
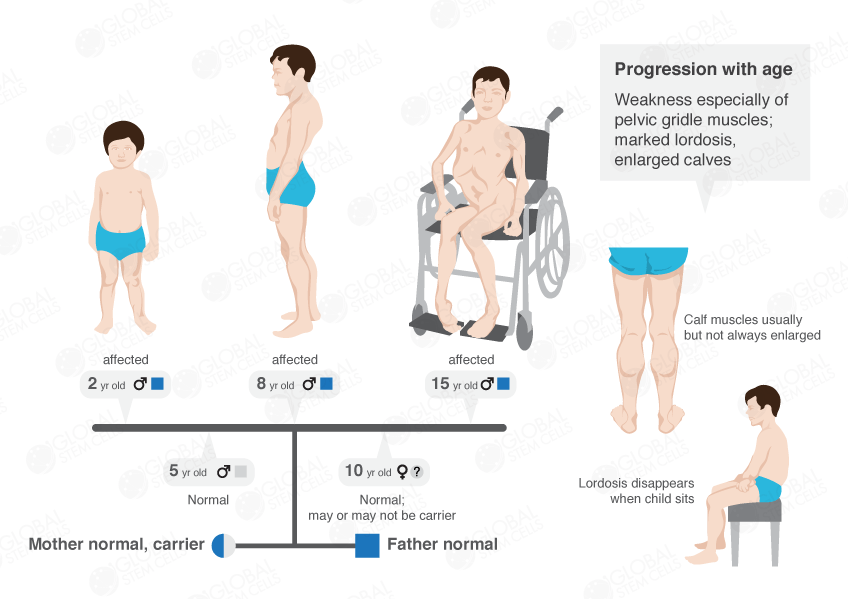
How Muscular Dystrophy Affects the Body
Duchenne Muscular Dystrophy (DMD) patients characteristically display progressive muscle weakness, which begins in early childhood. Although Duchenne Muscular Dystrophy (DMD) is present at birth, clinical symptoms are not evident until 3-5 years of age.
Initial symptoms of the illness include leg weakness, increasing convex curvature of the spine, and a waddle-like gait. Continuous muscle-wasting results in progressively weaker muscles, usually leaving DMD patients wheelchair-bound by the age of 11 or 12.
In the latter stages, most patients succumb to cardiac or respiratory failure in their twenties, although rare cases of survival into the thirties have been reported . A similar, yet milder, dystrophy known as Becker Muscular Dystrophy (BMD) is more variable phenotypically and generally follows a less severe course than Duchenne Muscular Dystrophy (DMD). Other types are generally adult onset and are comparatively slowly progressive.
The Very Best Stem Cell Treatments via globalstemcells.com
- Lapidos, Karen A.; Kakkar, Rahul; McNally, Elizabeth M. (2004)
“The Dystrophin Glycoprotein Complex Signaling Strength and Integrity for the Sarcolemma”. Circulation Research. 94 (8): 1023–1031. doi: 10.1161/01.RES.0000126574.61061.25. ISSN 0009-7330. PMID 15117830.
- Udd, Bjarne (2011).
“Distal muscular dystrophies”. Handbook of Clinical Neurology. 101: 239–62. doi: 10.1016/B978-0-08-045031-5.00016-5. PMID 21496636.
- Turner, C.; Hilton-Jones, D. (2010).
“The myotonic dystrophies: diagnosis and management”. Journal of Neurology, Neurosurgery & Psychiatry. 81 (4): 358–67. doi: 10.1136/jnnp.2008.158261. PMID 20176601.
- McAdam, Laura C.; Mayo, Amanda L.; Alman, Benjamin A.; Biggar, W. Douglas (2012).
“The Canadian experience with long term deflazacort treatment in Duchenne muscular dystrophy”. Acta Myologica. 31 (1): 16–20. ISSN 1128-2460. PMC 3440807. PMID 22655512.
- Verhaert, David; Richards, Kathryn; Rafael-Fortney, Jill A.; Raman, Subha V. (2011).
“Cardiac Involvement in Patients with Muscular Dystrophies: Magnetic Resonance Imaging Phenotype and Genotypic Considerations”. Circulation. Cardiovascular imaging. 4 (1): 67–76. doi: 10.1161/CIRCIMAGING.110.960740. ISSN 1941-9651. PMC 3057042. PMID 21245364.
- Laing, Nigel G; Davis, Mark R; Bayley, Klair; Fletcher, Sue; Wilton, Steve D (2011).
“Molecular Diagnosis of Duchenne Muscular Dystrophy: Past, Present and Future in Relation to Implementing Therapies”. The Clinical Biochemist Reviews. 32 (3): 129–134. ISSN 0159-8090. PMC 3157948. PMID 21912442.
- Bushby, Katharine; Finkel, Richard; Birnkrant, David J; Case, Laura (2010).
“Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management”. The Lancet Neurology. 9 (1): 77. doi: 10.1016/s1474-4422(09)70271-6. Retrieved 12 April 2016.
- Muntoni, F. (28 August 2001).
“Is a muscle biopsy in Duchenne dystrophy really necessary?”. Neurology. 57 (4): 574–575. doi: 10.1212/wnl.57.4.574. ISSN 0028-3878. PMID 11524463.
- Drug Safety and Availability. 2014
- Nigro, G (2010).
“One-hundred-seventy-five years of Neapolitan contributions to the fight against the muscular diseases”. Acta Myologica. 29 (3): 369–91. PMC 3146338. PMID 21574522.
- MedlinePlus Encyclopedia
External Links
- Muscular dystrophies at DMOZ
- CDC’s National Center on Birth Defects and Developmental Disabilities
(previously listed below as “Duchenne/Becker Muscular Dystrophy, NCBDDD”) at CDC - Genes and Disease Page at NCBI
Classification and External Resources
| Specialty | Pediatrics, medical genetics |
|---|---|
| ICD–10 | G71.0 |
| ICD–9-CM | 359.0–359.1 |
| MedlinePlus | 001190 |
| eMedicine | orthoped/418 |
| MeSH | D009136 |


 English
English