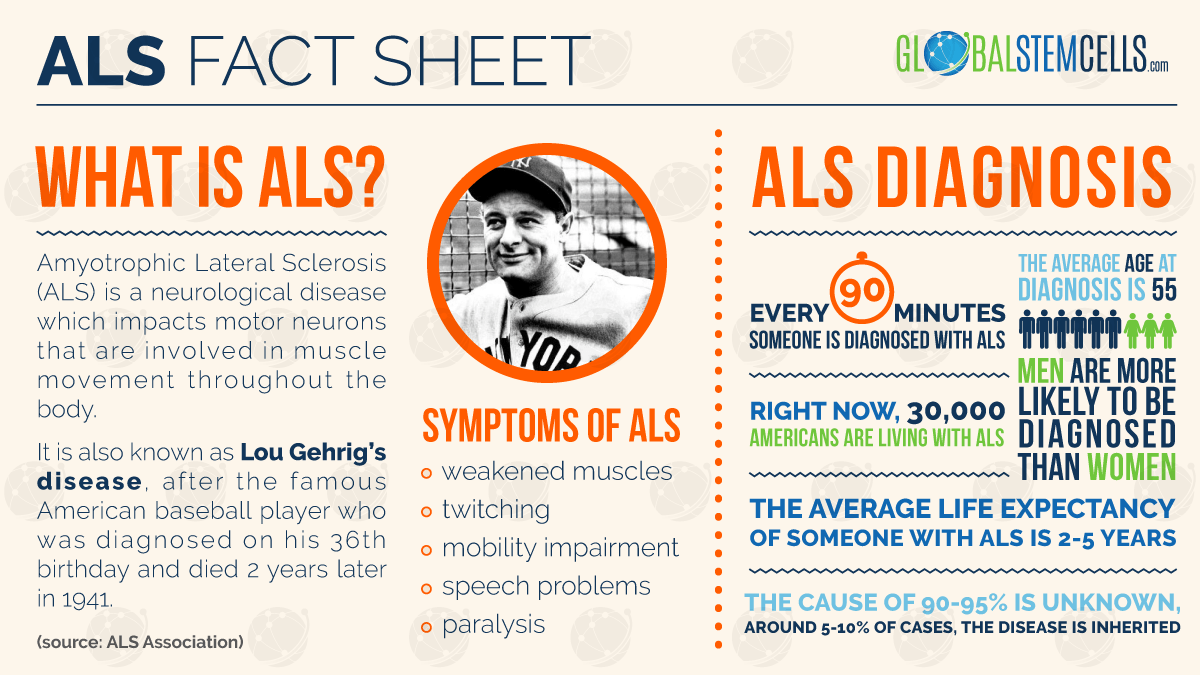
Amyotrophic Lateral Sclerosis (ALS), also known as Lou Gehrig’s disease is a rapidly progressive neurological disease that causes dysfunction of the nerves that control muscle movement. ALS is invariably fatal and the race to find a cure has been ongoing since the disease was first identified by French neurologist Jean-Martin Charcot in 1869. Stem Cell Therapy for Amyotrophic Lateral Sclerosis may soon be a reality in many countries of the world.
ALS Treatment and Symptoms
Treatment for ALS has so far recorded modest successes in slowing the progress of the disease and in managing the symptoms. These symptoms are wide-ranging and include progressive muscle weakness, unexplained fatigue of the arms and legs, slurred speech, tripping and dropping things. Muscle cramps and twitches are also closely associated with ALS. The disease is no respecter of persons and British physicist Stephen Hawking is perhaps its most famous living victim.
BrainStorm Cell Therapeutics an Israeli biotechnology company developing autologous stem cell therapies for neurodegenerative diseases has developed adult stem cell treatment for patients with ALS. BrainStorm has sought early approval in Canada for this treatment called NurOwn, an unusual step considering the fact that it has not completed late-stage clinical trials. Partnering with CCRM, a Canadian not-for-profit organisation that supports the development of regenerative medicine, BrainStorm seeks to fast-track approval for its ALS treatment by meeting the requirements of Canada’s health regulator’s early access pathway. This is a process that provides rapid review (and subsequent approval where the threshold is met) of drugs developed to treat both serious and life-threatening conditions. Advanced clinical trials for NurOwn are slated to begin in the second quarter of 2017 across multiple sites, both in Israel and in the United States.
All indications point to a quick review and approval of NurOwn by the Canadian Health authorities. BrainStorm has at the same time sought approval in Israel to allow patients to access NurOwn under the ‘Hospital Exemption’ protocol. BrainStorm Chief Executive Chaim Lebovits is confident that legislation passed in December 2016 in the US could also pave the way for early review and possible approval in the US itself. Lebovits is optimistic that treatments under the ‘Hospital Exemption’ pathway will commence as early as the second half of 2017.
Stem Cell Treatment for ALS Available Today
One does need to point out though that Stem Cell Therapy for ALS has been in use for some time now and instead of waiting for clinical trials those in need may seek treatment and obtain some relief from this deadly disease. The very fact that approval for Stem Cell Therapy appears to be a foregone conclusion in Canada is, of itself, a positive sign and should act as a goad to anyone considering treatment.
Many patients have seen remarkable improvements in their conditions after going through Stem Cell Therapy for Amyotrophic Lateral Sclerosis. These patients are enthusiastic about their progress and many are happy to share their stories.
Canada, Israel and the United States may quite soon join the more medically-progressive countries in the use of cutting edge technology and offer treatments and therapies which are scientifically proven and which help ease the lives of thousands suffering from debilitating diseases.
H/T: Reuters




 English
English